Prof. Peng's Group
PROFESSIONAL EXPERIENCE
Professor, Soochow University, China, 2016-present
Dean, College of Energy, Soochow University, China, 2024-present
Director, Soochow Municipal Laboratory for Low Carbon Technologies and Industries, China, 2021-present
Vice Dean, College of Energy, Soochow University, China, 2018-2024
Director, Key Laboratory of Advanced Carbon Materials and Wearable Energy Technologies of Jiangsu Province, China, 2017-2020
Senior Scientist- Upstream Oil & Gas Completion and Production, Halliburton, USA, 2013-2016.01
Research Associate, Joint Researcher of University of Pennsylvania & National Institute of Standards and Technology (NIST), USA, 2012-2013
Research Associate, University of Maryland College Park, USA, 2010-2012
Research Assistant, University of California, Davis, USA, 2005-2010
EDUCATION
Ph.D. in Inorganic Chemistry (Organometallic), University of California, Davis, U.S.A.
M.S. in Inorganic Chemistry, Nanjing University, China.
B.S. in Chemistry, Nanjing University, China.
HONORS
National Young Talents (Oversea).
Outstanding Dissertation Award of Chemistry Department at UC-Davis, 2010.
Chinese Government Award for Outstanding Student Self-financed Study Abroad, 2009-2010.
Research Interests
CO2 Catalysis Conversion Techniques
Our group was invited to write a perspectivearticle on “Electrochemical CO2 reduction catalyzed by organic/inorganic hybrids” in “eScience”. In this perspective, we summarize the recent progress on the design and fabrication of organic/inorganic hybrids CO2RR electrocatalysts, with special attention to the assembly protocols and structural configurations. We then carry out a comprehensive discussion on the mechanistic understanding of CO2RR processes tackled jointly by the inorganic and organic phases. We also outline future challenges in the rational design of organic/inorganic hybrids for CO2RR and further extend the scope to the device level.
For details, please refer to eScience. 2023,3,100097.
The hybrid inorganic/organic structures created a synergy to facilitate proton-coupled electron transfer (PCET) in CO2RR, so as to afford deeply reduced hydrocarbon products. Bridging nanostructured inorganic components with functional organic moieties offers a versatile approach to modify the electronic structure of active sites, tailor the local microenvironment at the gas/electrode/electrolyte interface, regulate the binding energies of reaction intermediates and ultimately steer the reaction pathway.

Design of Group IB Nanostructures
Breaking the Linear Scaling Relationship by Compositional and Structural Crafting of Ternary Cu-Au/Ag Nanoframes for Electrocatalytic Ethylene Production

For details, please refer to Angew. Chem. Int. Ed. 2021, 60, 2508–2518.
Geometric Modulation of Local CO Flux in Ag@Cu2O Nanoreactors for Steering the CO2RR Pathway

For details, please refer to Adv. Mater. 2021, 33, 2101741.
Metal-organic compounds
Metal-organic compounds (MOCs) have been well reckoned and extensively studied as promising catalysts or modulators for CO2RR owing to their high porosity, isolated active sites, tunable chemistry, as well as explicit structure for mechanistic understanding. However, under electrochemical conditions, especially at high current densities, their electric conductivity and structural stability impose a significant hurdle for practical applications. To circumvent the issue, MOCs were often utilized as the pre-catalysts to construct inorganic/organic hybrids for catalyzing CO2RR through in situ reduction. Combining both the merits from heterogeneous inorganic and homogeneous molecular catalysts, organic/inorganic hybrids endow great opportunities to rationally mediate the local CO2 supply and subsequent reaction cascade by modulating the catalyst architecture and local chemical environment. In virtue of the rich and tailorable functional groups, organic moieties in conjunction with conventional inorganic CO2RR electrocatalysts offer extra freedom to engineer the surface hydrophobicity/hydrophilicity, local pH, proton-electron synchronicity, adsorbate binding and thereby provides deep insights into the mechanisms of CO2 activation and electroreduction at molecular level.
Cu crystallites induced and stabilized via current shock and charge delocalization

The effect of conducting support on the CO2RR behaviors of semi-conductive metal-organic framework (MOF) - Cu3(HITP)2 are carefully investigated. Compared to the stand-alone MOF, adding Ketjen Black greatly promotes C2H4 production with a stabilized Faradaic efficiency between 60-70% in a wide potential range and prolonged period.
For details, please refer to Nat. Commun. 2021, 12, 6823.
Modulating the molecular geometry and Cu coordination in bicentric copper complexes

Inorganic and organic phases to synergistically produce alcohols, of which the intermediates are stabilized by a confined space to afford extra O-Cu bonding.
For details, please refer to Nat. Commun. 2022, 13, 5122.
Au-activated N motifs in non-coherent cupric porphyrin metal organic frameworks for promoting and stabilizing ethylene production

Au nanoneedles inserted into the MOF can effectively bypass the charge flow going through the non-coherent framework and thereby improve both the catalyst conductivity and stability. Surprisingly, despite the lack of a coherent structure, the Au-inserted framework affords a superb ethylene selectivity up to 52.5% in Faradaic efficiency, ranking among the best for metal-organic frameworks reported in the literature.
For details, please refer to Nat. Commun. 2022, 10, 3782.
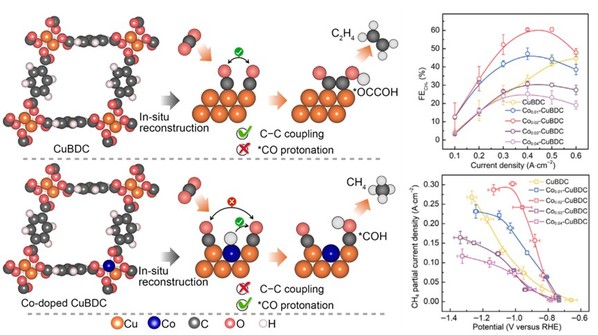
The fabrication of an atomically dispersed Co-Cu alloy through the reconstruction of trace-Co doped Cu metalorganic framework. The introduction of atomically dispersed Co in Cu favors *CO protonation via enhancing surface water activation, and suppresses C−C coupling by reducing *CO coverage, thereby leading to high methane selectivity. Finally, the methane yield with a current of up to 500 mA/cm2 and a Faraday efficiency of up to 60.5 %.
For details, please refer to Nano Res. 2023, 16, 3680-3686.
Reactor Design
H-Cell, Flow Cell
The productivity and energy efficiency of CO2RR isnotoriously limited by the low solubility of CO2 in aqueous electrolyte and high ohmic loss in H-type and flow cells. Taking advantage of the fast CO2 mass transport, conventional gas diffusion electrodes (GDEs) with the laminate configuration greatly boost up the production rate of CO2RR, but still suffers from poor interfacial stability and limited utilization of active sites, in addition to issues of flooding and salt precipitation.

MEA: Integral Gas Diffusion Electrode for Full-pH CO2 Electroreduction

In virtue of the integral architecture, hierarchical porosity and highly active catalytic sites, the optimized GDE showcases a near-unity Faradaic efficiency of CO and stable operation for more than 273 hours with a total energy efficiency of 38% in neutral MEA, as well as a high single-pass CO2 conversion (SPCCO2) of 78% in acidic MEA. Through the structural innovation of GDE and detailed analysis on the failure mechanism, this work paves the way for industrial-scale CO2 electrolysis.
For details, please refer to https://doi.org/10.1039/D3EE01866K.
Research Interests
In-situ/Operando Electrochemical Characterization
Modern spectroscopy techniques including Raman, infrared, X-ray absorption and differential electrochemical mass spectrometry, can track the chemical reaction kinetics in supreme space, time and spectral resolutions. Dynamic Raman spectra allow one to obtain delicate information about intermediates configuration and local environment, while FTIR spectroscopy of high sensitivity and fast speed enables to resolve transient evolution of intermediates and chemical bondings. In-situ XAS is potent at tracing the changes of valence and coordination states of metal active sites, offering profound insights into the dynamic evolution of ligated metal centers. Differential electrochemical mass spectroscopy (DEMS) enables to conduct quantitative analysis of gaseous products and volatile intermediates with applied bias in a remarkable time resolution. By analyzing trace gaseous intermediates generated or consumed in the process of CO2RR, the reaction pathway regulated by organic/inorganic hybrids might be mapped out. By in-situ electrochemical spectroscopy, we can study the surface structure of electrocatalyst and the surface process of electrocatalytic reaction at the atomic and molecular level, which is helpful to understand the mechanism of catalytic active site, and promote the practical application of electrocatalyst.

